PROTECT YOUR DNA WITH QUANTUM TECHNOLOGY
Orgo-Life the new way to the future Advertising by Adpathway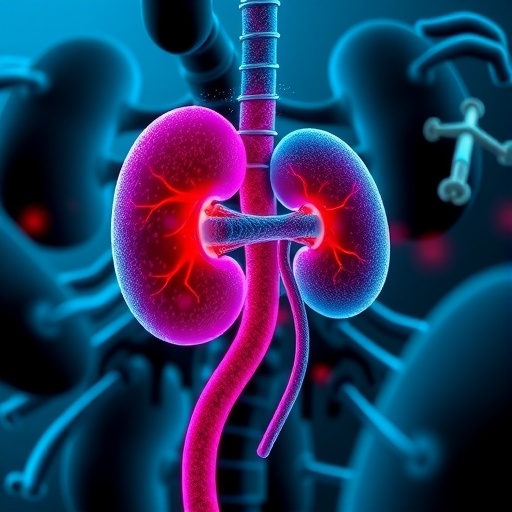
Emerging research from the University of Illinois Urbana-Champaign in collaboration with Mie University in Japan has uncovered a pivotal role for a gut bacteria-produced molecule in driving diabetic kidney fibrosis, a leading cause of kidney failure worldwide. This molecule, known as corisin, is a small peptide secreted by Staphylococcus species residing in the human gut microbiota. Scientists have revealed that corisin can traverse the complex biological pathways from the intestine to the kidneys, where it triggers a cascade of inflammatory events culminating in cellular aging, tissue scarring, and ultimately, diminished renal function.
Kidney fibrosis caused by diabetes remains an elusive and formidable challenge for medical science. This scarring process progressively replaces functional kidney tissue with fibrotic deposits, severely impairing the organ’s ability to filter blood and maintain homeostasis. Until now, the precise molecular actors orchestrating this pathological progression remained largely unidentified, leaving clinicians without targeted therapeutic options. The collaborative research team centered their investigation on the hypothesis that microbial peptides, such as corisin, might be covert drivers of this fibrogenic process.
Initial clinical observations involved rigorous screening of blood and urine samples from patients with diabetic kidney disease. These tests revealed a compelling correlation: elevated blood levels of corisin tightly aligned with worse kidney damage among patients suffering from diabetic nephropathy. Intriguingly, these findings were mirrored in a mouse model genetically and pathologically engineered to mimic the human disease. The reproducibility of corisin’s presence and impact across species underscored its potential as a universal contributor to kidney fibrosis.
Mechanistically, the researchers employed a multidisciplinary approach combining in vivo animal experiments, histopathological tissue analyses, and sophisticated computational simulations to dissect corisin’s biological trajectory. Computer models revealed that corisin attaches to albumin, the most abundant plasma protein, facilitating its transport through the bloodstream. This hitchhiking phenomenon allows corisin to bypass immunological detection and physiological barriers, reaching the intricate microenvironments within the kidneys where filtration occurs.
Upon arriving at the renal filtration apparatus, corisin detaches from albumin and begins to interact directly with kidney cells, particularly those that form the glomerular and tubular structures responsible for urine production. The peptide accelerates cellular aging by inducing senescence pathways, triggering increased production of pro-inflammatory cytokines and reactive oxygen species. This cellular stress precipitates apoptosis and stimulates fibroblast activation, leading to extracellular matrix accumulation and progressive scarring.
The pathological alteration does not stop at the cellular level but extends to reshape the kidney microarchitecture. Fibrotic tissue replaces functional nephron units, compromising filtration efficiency and gradually manifesting as chronic kidney disease symptoms. These irreversible changes underscore the critical necessity of identifying upstream modulators like corisin to interrupt the fibrosis cascade early.
To validate the causative role of corisin, researchers designed experiments administering antibodies specifically targeting the peptide to affected mice. Treatment with these neutralizing antibodies markedly decelerated the progression of fibrosis, suppressed markers of cellular senescence, and improved renal function metrics. Although these antibody therapeutics have not yet advanced to human trials, the proof-of-concept highlights a promising new avenue for therapeutic intervention designed to halt or even reverse diabetic kidney fibrosis.
Besides demonstrating therapeutic potential, the study expands our understanding of the gut-kidney axis, an emerging concept describing how intestinal microbiota influence distant organ systems. This research exemplifies the systemic implications of microbial metabolites, positioning corisin as a pathogenic communicator that converts gut microbial activity into debilitating kidney damage. Such insights open exciting possibilities for microbiota-targeted interventions to mitigate complications of metabolic diseases.
To further elucidate corisin’s impact and therapeutic feasibility, future studies plan to utilize more advanced animal models like swine, which better mimic human organ physiology and drug metabolism. These larger models will facilitate evaluation of antibody safety, pharmacodynamics, and dosing regimens, bridging the critical translational gap from bench to bedside.
The research team acknowledges the complexity of diabetic kidney fibrosis but remains optimistic that targeted anti-corisin therapies, whether antibodies or alternative molecular inhibitors, could dramatically improve outcomes for millions afflicted by diabetes-induced renal impairment. By shifting treatment paradigms from symptomatic management to molecular interception, this discovery inaugurates a new chapter in nephrology research and raises hope for innovative cures.
The study, published in the prestigious journal Nature Communications, reflects a robust interdisciplinary collaboration. It interweaves microbiology, immunology, chemical engineering, and clinical sciences to unravel a fundamental pathological mechanism and propose a viable therapeutic strategy. As the research progresses, it may open corridors toward microbiome-informed precision medicine approaches for chronic kidney disease and possibly other fibrosis-linked conditions.
In summary, this groundbreaking work elucidates how corisin—a microbial peptide formerly flying under the radar—plays a dominant role in accelerating kidney fibrosis by fostering premature senescence within renal cells. With scientific rigor and technological innovation, the research not only highlights a hidden perpetrator in diabetic kidney damage but also positions corisin blockade as a promising frontier toward transforming patient care.
Subject of Research: Animals
Article Title: Microbiota-derived corisin accelerates kidney fibrosis by promoting cellular aging
News Publication Date: 25-Aug-2025
Web References:
https://www.nature.com/articles/s41467-025-61847-2
References:
Cann, I., Gabazza, E., et al. (2025). Microbiota-derived corisin accelerates kidney fibrosis by promoting cellular aging. Nature Communications. DOI: 10.1038/s41467-025-61847-2
Keywords:
Diabetic kidney fibrosis, corisin, gut microbiota, Staphylococcus peptide, kidney inflammation, cellular senescence, fibrosis, antibody therapy, microbiota-kidney axis, renal aging, nephropathy, albumin transport, therapeutic development
Tags: corisin peptide and diabetesdiabetic kidney fibrosis researchfibrosis and tissue scarring in kidneysgut bacteria and kidney healthinflammatory processes in kidney diseasekidney failure and gut-derived moleculesmolecular mechanisms of kidney fibrosisrenal function and gut microbiomerole of microbiota in diabetic complicationsStaphylococcus species in gut microbiotatherapeutic targets for kidney fibrosisUniversity of Illinois kidney research


 4 hours ago
10
4 hours ago
10


















 English (US) ·
English (US) ·  French (CA) ·
French (CA) ·